Magnesium is a highly reactive metal which reacts violently with oxygen to produce a white solid called magnesium oxide. This can be shown as fo...
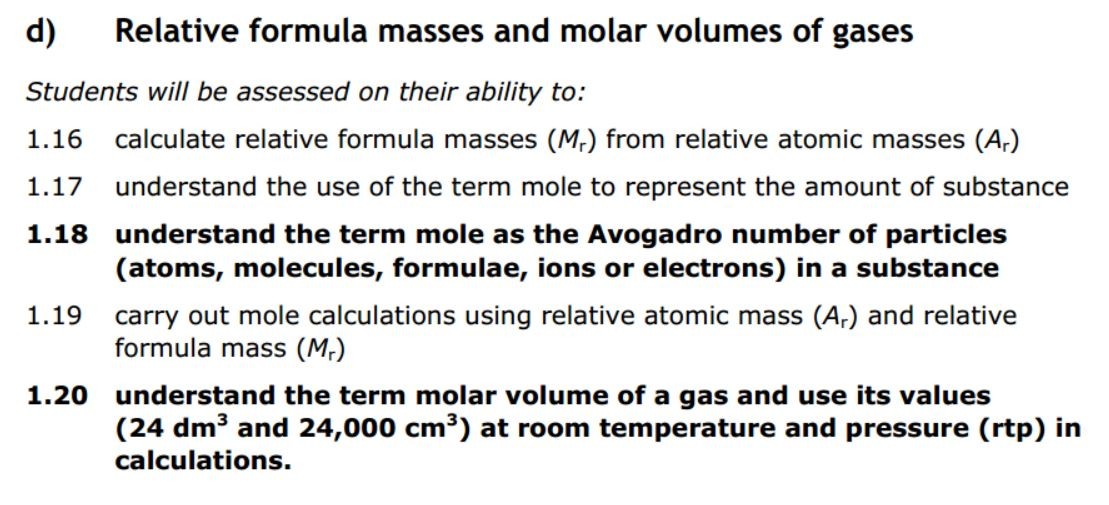
1.27 What is a mole? Students should: 1.27 know that the mole (mol) is the unit for the amount of a substance 1.28 understand how to carry...
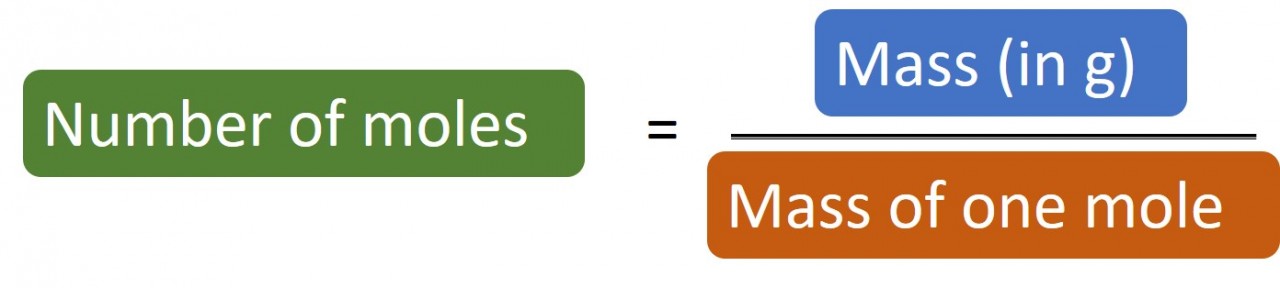
1.28 Calculating moles The following masses of elements all contain one mole of atoms: 12.0 g Carbon, 32.1 g Sulphur, 14 g Nitrogen,&n...
Relative masses allow chemists to "count out" atoms and molecules so they can ensure that the appropriate amounts of substances are reacted.
1.26 "Counting" by measuring mass Sometimes chemists need to "count" out a specific number of atoms, ions or molecules of a substance....
Relative masses allow chemists to "count out" atoms and molecules so they can ensure that the appropriate amounts of substances are reacted.
Concentration Noun :the action or power of focusing all one's attentiona close gathering of people or things.the relative amount of a particular...
Finding the formula of a compound is important to chemists. We can often predict what the formula might be because we know something about the el...
In this section we consider how much product is formed by a chemical reaction . Some reaction processes are not very efficien...