Structure and bonding
in this topic, we have looked at the way in which substances
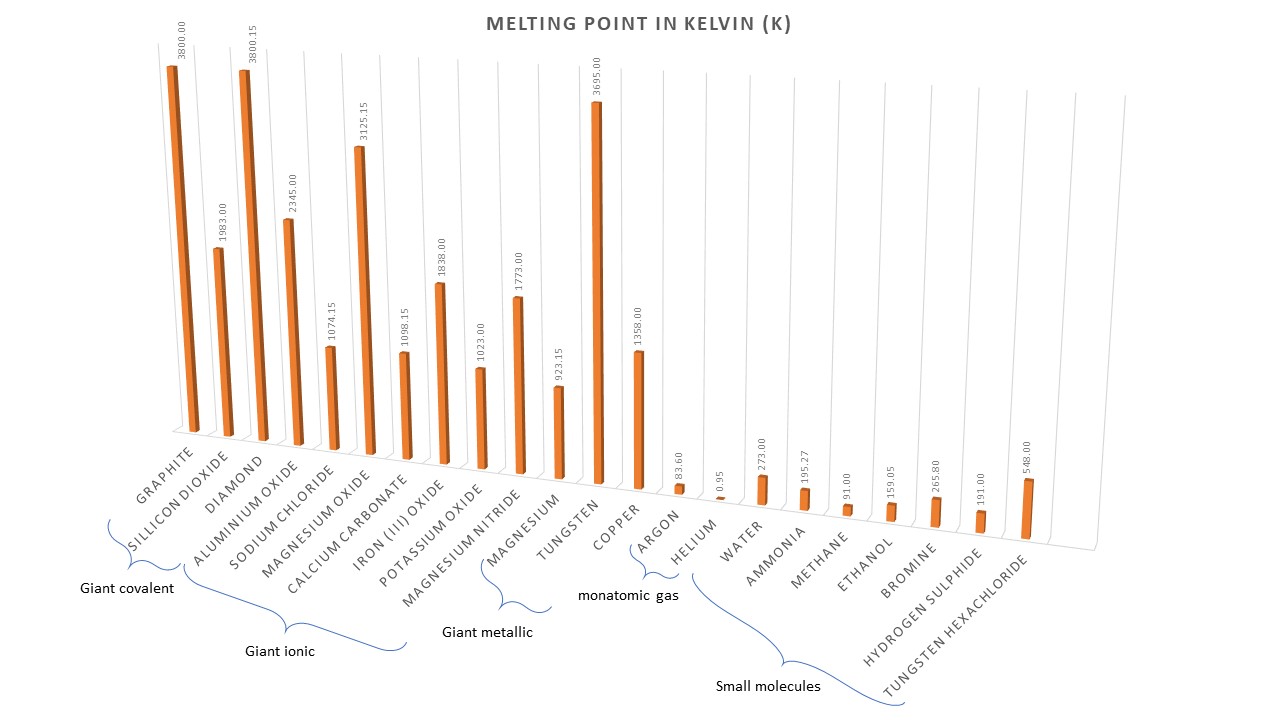
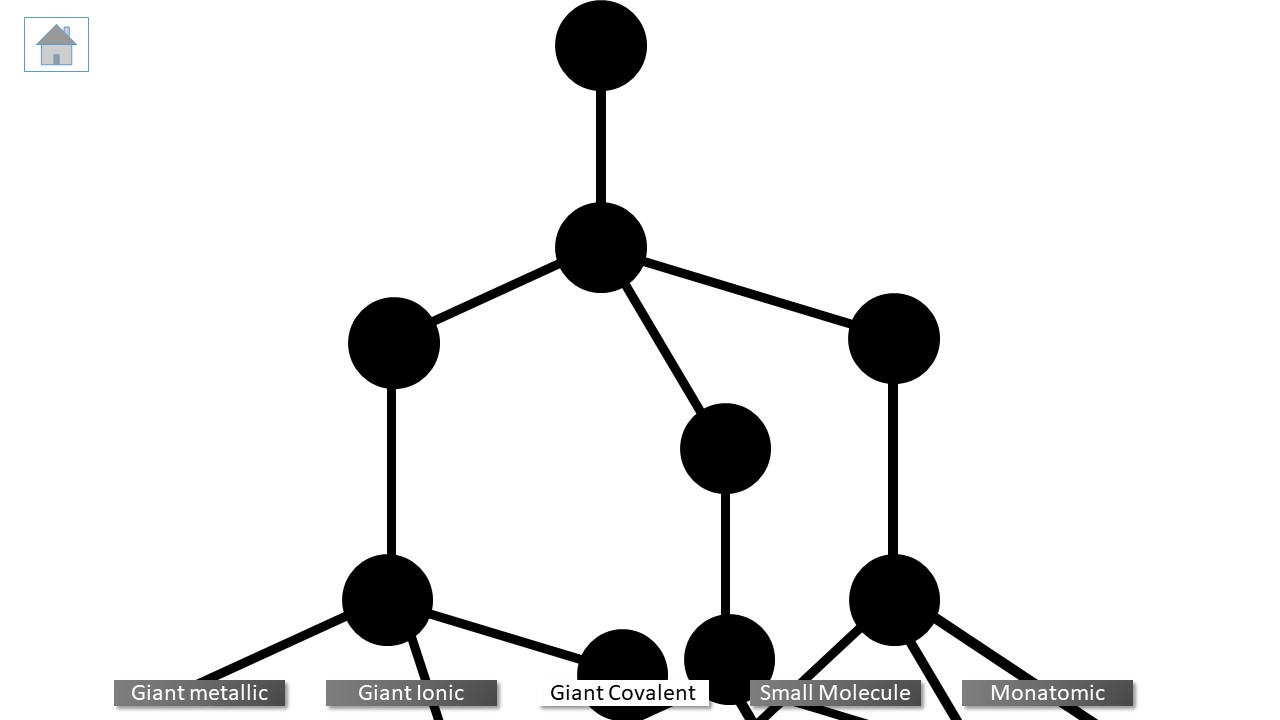
Three types of bonding are. metallic, covalent and ionic.
metallic: formed when metals bond with themselves or other metals.
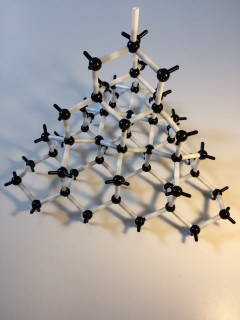
covalent: formed when non-metal bonds with itself or another non-metal.
ionic: formed when metals bond with non-metals.
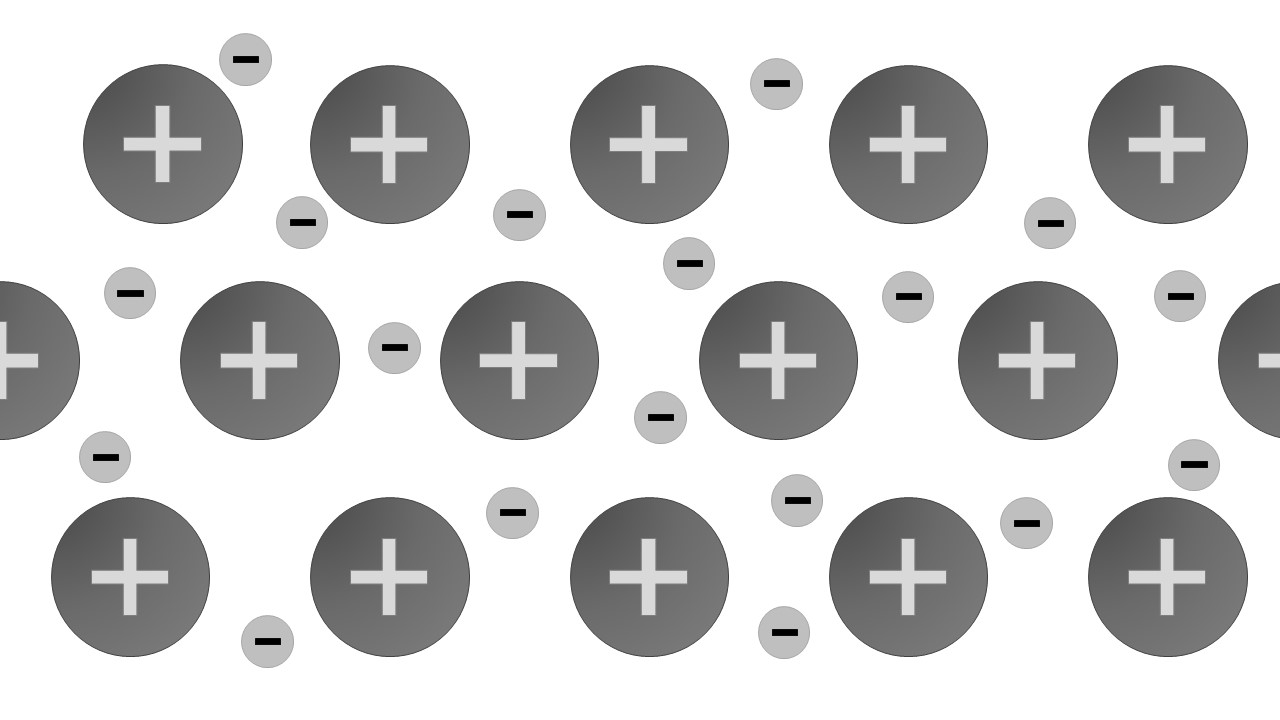
Giant metallic lattices
General properties
- all conduct electricity when solid and molten
- most are malleable
- many have high melting point
Description
Positive ions form an orderly arrangement which is held together by the attraction of the delocalised electrons .
Formation
Formed in any pure metal and when one metal mixes with another metal to form an alloy
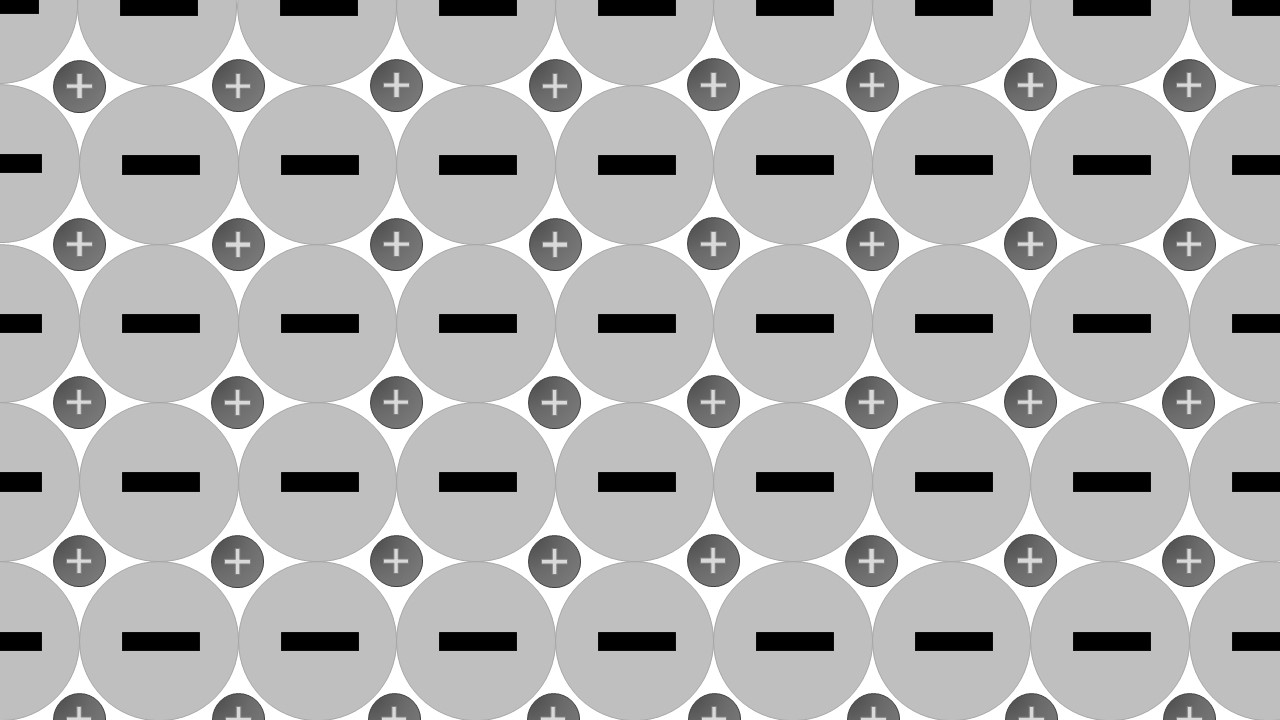
Giant ionic lattices
Enter your text here ...
Properties
- conduct when molten
- high melting points
- crystalline
- can be soluble in water
Description
Positive ions are attracted to and surround negative ions. Negative ions are attracted to and surround positive ions. A three-dimensional lattice forms.
Formation
Formed when metals combine with non-metals . The metals form positive ion which are attracted to the negatively charged non-metal ions
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.