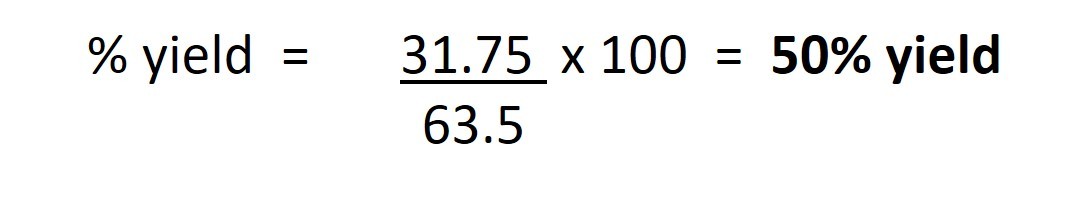1.30 Percentage yield
In this section we consider how much product is formed by a chemical reaction . Some reaction processes are not very efficient and only a small proportion of the reactants are actually turned into the products.
The yield of a chemical reaction is the amount of product obtained.
It is usually expressed as a percentage.
The percentage yield is calculated by dividing the actual "practical" amount produced ( actual yield) by theoretical yield.
1.30 Activity 1. Understanding percentages
Use the animation to see what is meant by percentage yield in terms of particles. Make a copy of the table and add the missing data.
Students should:
1.30 calculate percentage yield
| percentage yield | no. reactant particles | no. product particles |
| 0 | 20 | 0 |
| 10 | | |
| 30 | | |
| 50 | | |
| 70 | | |
| 90 | | |
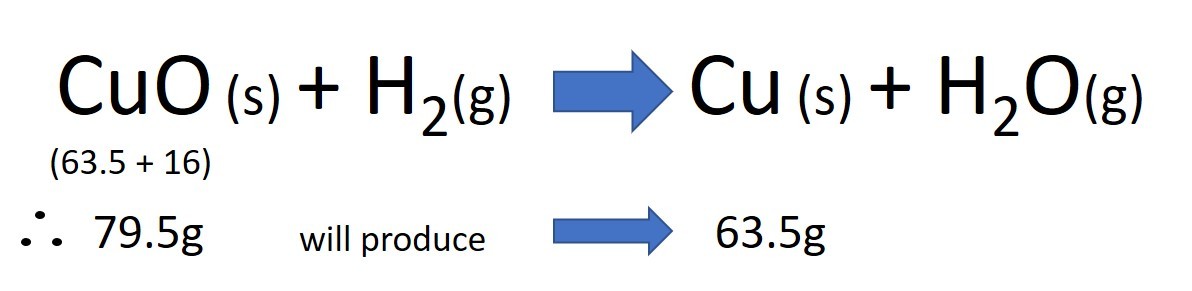
The theoretical yield is usually calculated using the balanced equation for the reaction and the relative masses of the substances involved. For example in theory if the reaction is complete and no product is lost or spilt then...
In this example, the theoretical yield of copper would be 63.5g. If the amount of copper actually produced from 79.5g copper oxide was 31.75g then the actual yield would be 31.75g. Thus the % yield would be: