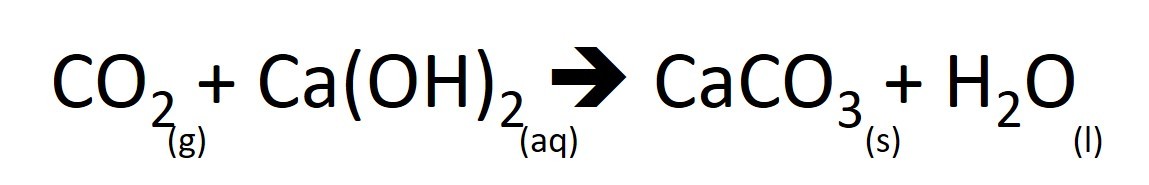2.44 Tests for gases
Physically gases have a number of similarities. For example - all gases will:
- fill the container in which they are placed and adopt its shape
- expand when heated and contract when cooled
- be compressed if subjected to increased pressure
One of the gases is alkaline one is acidic, one burns, another supports combustion and the other acts as a bleach. Here you will find out which does what.
Students should:
- 2.44 describe tests for the gases:• hydrogen • oxygen • carbon dioxide • ammonia • chlorine.
2.44 Activity 1. Observing Oxygen
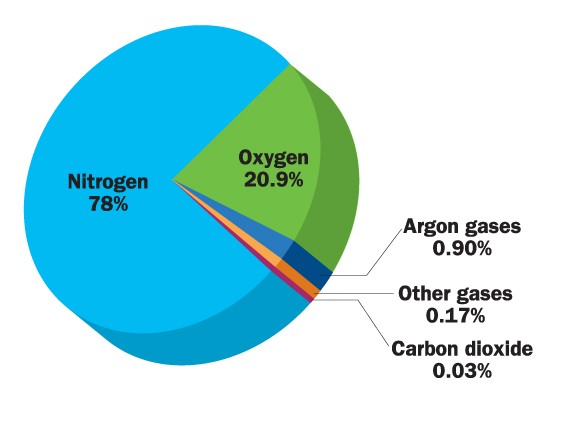
Oxygen is a very reactive gas. It makes up approximately 21% of our atmosphere.
Oxygen is a gas which supports combustion. If the proportion of oxygen in the air is increased then things will burn more readily. An atmosphere any richer in oxygen would make the world a more dangerous place!
The standard test for oxygen is to place a glowing splint into a test tube that may contain the gas.
If the splint glows brighter and/or relights into flame then it is a positive result for oxygen.
2.44 Activity 2. Hearing Hydrogen
This video shows how when done on a larger scale hydrogen will burn with a bit more than a squeaky pop!
2.44 Activity 3. Catching carbon dioxide
2.44 Activity 4. Detecting Ammonia gas
Ammonia gas is a colourless but very pungent gas. Ammonia gas is manufactured on an industrial scale - as it can be used as a fertiliser itself and neutralised by acids to make salts which can be used as fertilisers. Other products include dyestuffs and explosives.
Ammonia can be easily detected using damp red litmus paper. The litmus paper turns blue as the ammonia is alkaline.
Pure ammonia is dangerous
2.44 Activity 5. Keep clear of Chlorine
Enter your text here ...
Chlorine is such an unpleasant gas that is has been used as a chemical weapon.
2.44 Activity 6. It's a gas
Make a copy of the table below. Watch the video and use the information to complete the table.
| GAS | TEST | POSITIVE RESULT |
| Oxygen | ||
| Hydrogen | ||
| Ammonia | ||
| Chlorine | ||
| Carbon dioxide |
| GAS | TEST | POSITIVE RESULT |
| Oxygen | ||
| Hydrogen | ||
| Ammonia | ||
| Chlorine | ||
| Carbon dioxide |
Enter your text here ...