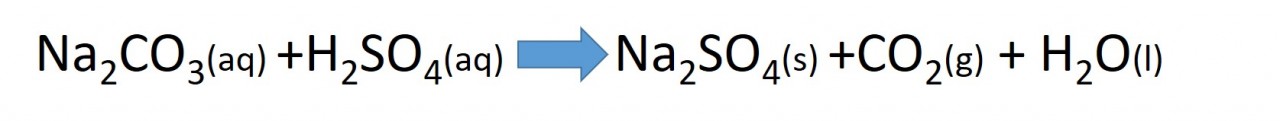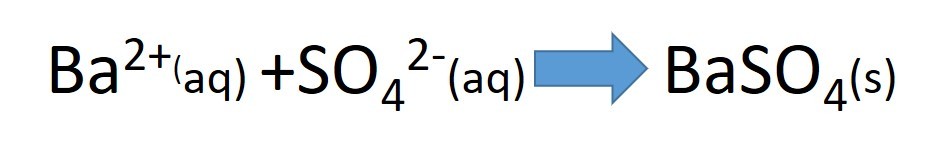2.45 - 2.50 Chemical tests (for ions)
2.45 - 2.46 Up in flames
Fireworks produce a whole range of different colours. All fireworks release energy in the form of heat energy and are therefore exothermic. Explosions and rocket power is provided by gunpowder and similar explosive mixtures.
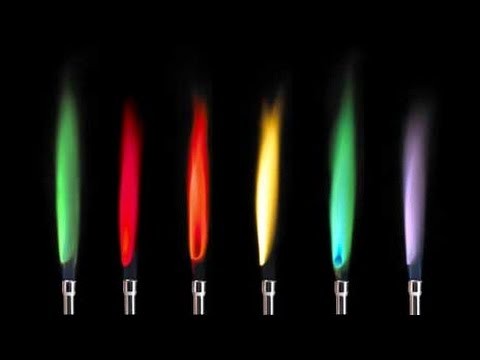
Colours are achieved by mixing compounds which contain various metal ions into the explosive. When hot, these metal ions produce light of specific colours. The colour produced by a particular metal ion can be used in the laboratory as way of identifying the metal ion present in a compound.
Assumed background knowledge

1.37 - 1.39 Ionic bonding - transferring charge
2.45. Activity 1
Students should:
- 2.45 describe how to carry out a flame test
Study the "positive ions part 1" video
- Use the video to write a sequence of numbered instructions which would allow a friend to successfully carry out a flame test on an unknown substance.
- outline the safety precautions which you should take
- explain why it is important to clean the nichrome wire thoroughly for each substance
How to perform a flame test
2.46 Activity 2. Colour coding - to learn
Students should:
- 2.46 know the colours formed in flame tests for the cations: lithium, sodium, potassium, calcium, copper
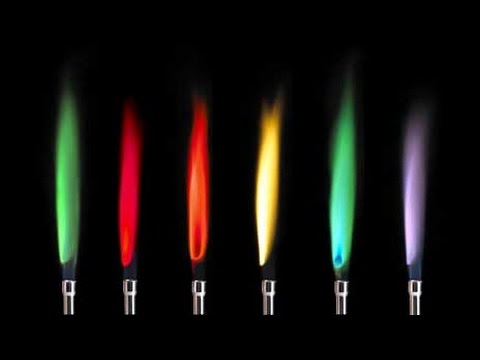
Flame emission spectroscopy
| Metal cation | flame colour |
| lithium | red ("crimson") |
| potassium | lilac |
| calcium | orange ("brick") red |
| sodium | yellow |
| copper | blue-green |
| barium | green |
2.47 Activity. Testing for some other Cations:
Students should:
- 2.47 describe tests for these cations: Ammonium, Copper II, Iron II and Iron III.
Use the video find out the results obtained when sodium hydroxide solution is added to solutions containing either ammonium (NH4+) ions, Copper (II) ions (Cu2+), Iron II ions (Fe2+) or iron III ions (Fe3+) . Add your results to a copy of the table
| Name of ion | Formula | Result observed |
| Name of ion | Formula | Observation |
| Ammonium | NH4+ | Warming with sodium hydroxide produces an alkaline gas |
| Copper(II) | Cu2+ | blue precipitate produced |
| Iron(II) | Fe2+ | green precipitate produced |
| Iron(III) | Fe3+ | brown precipitate produced |
The positive result for the presence of ammonium ions is when warmed with sodium hydroxide solution, ammonia gas is produced. This is detected using damp red litmus paper which turns blue in the presence of the alkaline gas ammonia.
2.48 Activity. Testing for anions: Carbonate ions
Students should:
- 2.48 describe tests for these anions: chloride, bromide, iodide, sulfate and carbonate
The test for carbonate ions is to add an acid to the unknown substance. Bubbles of carbon dioxide will be formed if a carbonate is present.
The equation below is for the reaction of sodium carbonate with sulfuric acid.
The reaction shown in the video uses calcium carbonate as the carbonate being tested.
The acid used is hydrochloric acid.
Use the equation above and the fact that the reactants used in the video are calcium carbonate and hydrochloric acid to help you predict the full symbolic equation for the reaction in the video.
How do they prove that the gas is carbon dioxide?
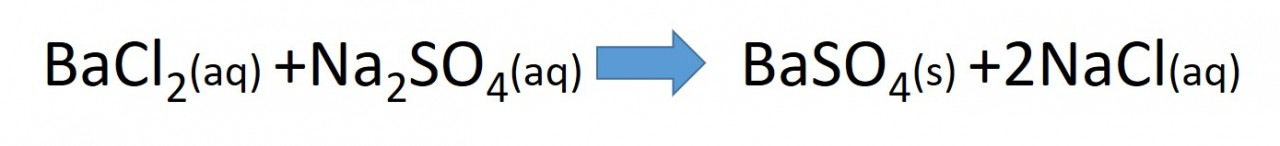
2.48 Activity. Testing for anions: Sulfate ions
The insolubility of barium sulfate is why barium ions are used to test unknown solutions for the presence of sulfate ions. Acidified barium sulfate solution is added to the unknown solution.
A white precipitate indicates the presence of sulfate.
2.48 Activity. Testing for anions: chloride, bromide and iodide ions
Acidified silver nitrate solution is added to the unknown solution. A precipitate forms if chloride, bromide or iodide are present.
This test for chloride ions bromide and iodide ions uses the fact that silver chloride , silver bromide and silver iodide are all insoluble in water.
Silver chloride is white, silver bromide is cream and silver iodide is yellow.
Ammonia solution will dissolve silver chloride but not silver iodide.