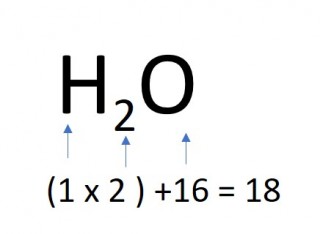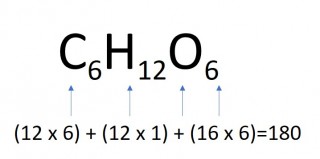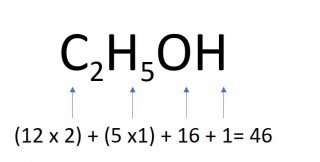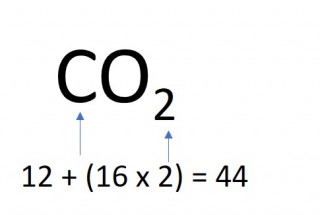1.26 - 1.28 Moles and mountains
1.26 "Counting" by measuring mass
Sometimes chemists need to "count" out a specific number of atoms, ions or molecules of a substance. Atoms are very very small - too small to see. It is therefore impossible to measure them out by counting individually. Instead we "count" them out by measuring their mass - much like the way in which coins are counted out in banks by using weighing machines.
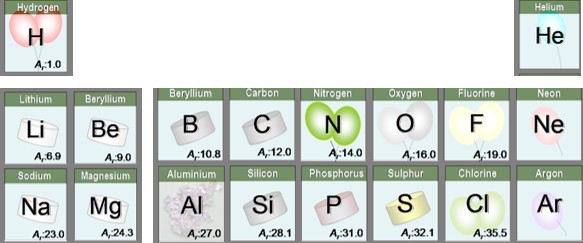
We know the Relative atomic masses ( or Ar) of atoms. This is usually shown next to the symbol of the element on the periodic table.
Assumed background knowledge:

1.14 - 1.17 Atomic structure
Students should:
1.25 write word equations and balanced chemical equations (including state symbols):
- for reactions studied in this specification
- for unfamiliar reactions where suitable information is provided
1.26 calculate relative formula masses (including relative molecular masses) (Mr) from relative atomic masses (Ar)
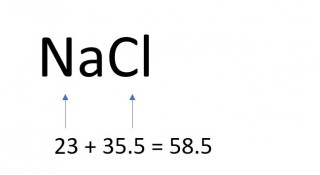
1.26 Calculating formula mass
The mass of one mole of a compound can be calculated using the relative atomic masses of the component elements multiplied by the number of atoms shown in the formula.
1.27 What is a mole?
Students should:
1.27 know that the mole (mol) is the unit for the amount of a substance
1.28 understand how to carry out calculations involving amount of substance, relative atomic mass (Ar) and relative formula mass (Mr)
A mole (mol) is the quantity of anything that has the same number of particles as there are atoms found in 12.0 grams of carbon-12.
This number of particles is a very large number : 6 x 1023
One mole of atoms of an element is the amount of that element equal to the relative atomic mass in g.
In other words - one mole of oxygen atoms has a mass of 16g, one mole of Lithium atoms has a mass of 6.9g etc.
Thus there are the same number of atoms in 16g of oxygen as there are atoms in 6.9g Lithium and of course 12g of Carbon.
1.28 Calculating moles
The following masses of elements all contain one mole of atoms:
- 12.0 g Carbon,
- 32.1 g Sulphur,
- 14 g Nitrogen,
- 24.3 g Magnesium.
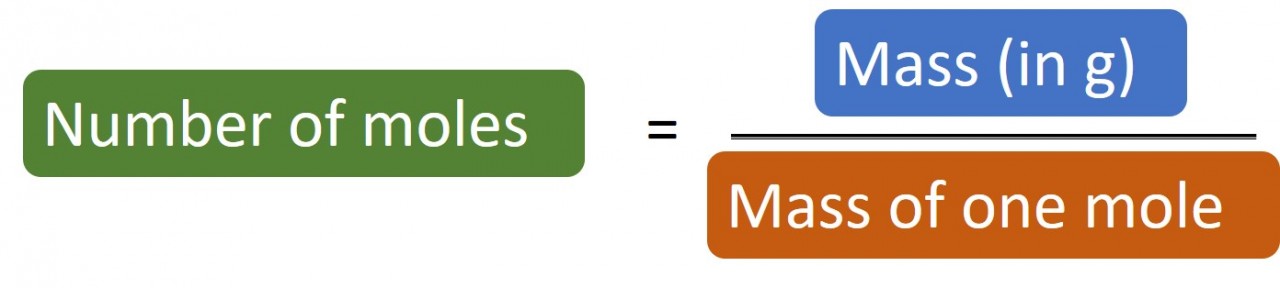
So if you have 24 g carbon you have 24/12 = 2 moles carbon atoms.
Likewise if you have 1.4 g nitrogen you have 1.4/14 = 0.1 moles nitrogen atoms.
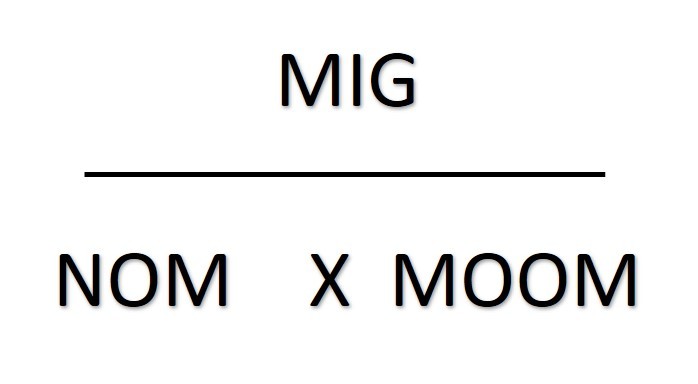
Rearranging things
The mass of one mole ("MOOM")is the relative atomic number for an element. If the substance is a compound you will need to work out its relative molecular mass or relative formula mass.
Activity 1. Moles from mass
Watch the short video to see how the number of moles can be calculated from the mass of the sample and the mass of one mole (Ar) . Use the same method to calculate the number of moles in the five samples given on the questions tab below. Use the answers tab to check your calculations:
- 355g of chlorine
- 0.69g of lithium
- 8.0g of oxygen
- 35g of copper
- one tonne of lead
There are:
- 10
- 0.1
- 0.5
- 0.55
- 4826.25
Activity 2. Mass from moles
Watch the short video to see how the mass of a sample can be calculated from the number of moles and the mass of one mole (Ar) . Use the same method to calculate the mass in the ten samples given on the questions tab below. Use the answers tab to check your calculations:
What is the mass of:
- 2.5 moles of oxygen atoms?
- 0.15 moles boron atoms
- 5 moles of aluminium atoms
- 0.4 moles of sulfur atoms
- 50 moles of oxygen atoms
- 1 mole of water molecules
- 0.5 moles of ethanol
- 4 moles of glucose
- 2 moles of sodium chloride
- 2 moles of carbon dioxide molecules
Enter your text here ...
Enter your text here ...
Enter your text here ...
Enter your text here ...
Enter your text here ...
- 2.5 x 16 = 40g
- 0.15 x 10.8 = 1.62g
- 5 x 27 = 135g
- 0.4 x 32.1 = 12.84g
- 50 x 16 = 800g
- 1 x 18 = 18g
- 0.5 x 46 = 23g
- 4 x 180 = 720g
- 2 x 58.5 = 117g
- 2 x 44 = 88g
Activity 3. Matching moles and masses
When you have successfully completed the calculations in activities 1 and 2 you should be able to test your skill using the quizlet below: