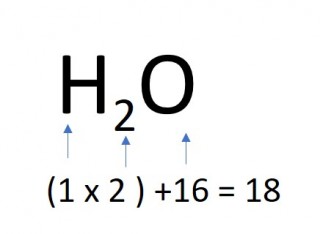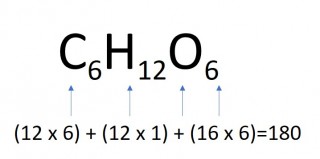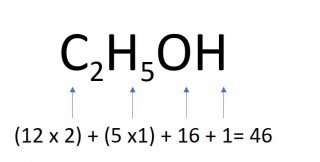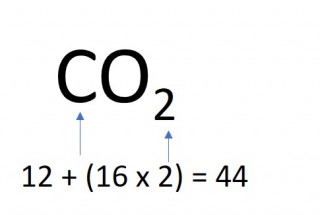1.26 Formula masses
1.26 "Counting" by measuring mass
Sometimes chemists need to "count" out a specific number of atoms, ions or molecules of a substance. Atoms are very very small - too small to see. It is therefore impossible to measure them out by counting individually. Instead we "count" them out by measuring their mass - much like the way in which coins are counted out in banks by using weighing machines.
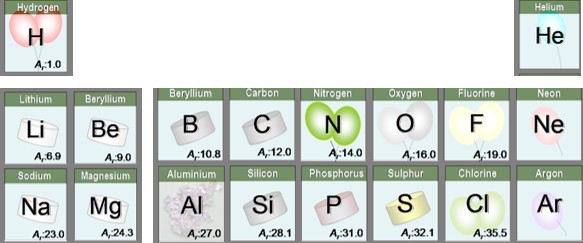
We know the Relative atomic masses ( or Ar) of atoms. This is usually shown next to the symbol of the element on the periodic table.
Assumed background knowledge:

1.14 - 1.17 Atomic structure
1.26 Calculating formula mass
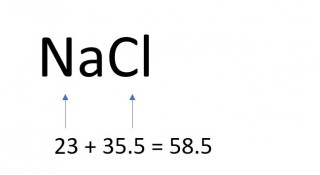
The mass of one mole of a compound can be calculated using the relative atomic masses of the component elements multiplied by the number of atoms shown in the formula.
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.