Chirality : left hand / right hand?

Chemistry Nobel awarded for mirror-image molecules - BBC News
Year 13 chemistry students will find out that molecules can be "handed". This is the result of property known as chirality.
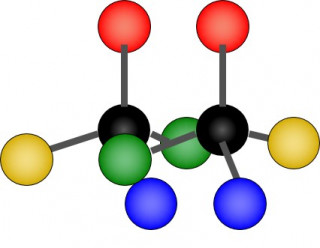
When a carbon atom within a molecule is bonded to four different groups ( e.g. methyl, hydrogen, amino, and carboxylic acid) the molecule can exist in two different forms (enantiomers) . Each enantiomer is a mirror image of the other.
The two forms (enantiomers) of the same molecule are almost identical but cannot be superimposed on one another. They are non-superimposable mirror images.
Explaining polarisation
Richard Thornley explains what plane polarised light is and how enantiomers have equal but opposite effects on its rotation.
Measuring rotation
Using two polarising filters. When two polarising filters are set at 90 degrees to one another - no light passes through.
If an optically active solution is placed in the polarimeter, the plane of the light is rotated and light can be seen by the observer. The movable filter is rotated until no light is seen and the angle of rotation measured.
Amino acids
Naturally occurring amino acids ( with the exception of glycine) all possess a chiral carbon atom. This means that there are at least two possible forms of these molecules. The two forms are given the label L (left handed) or D (right handed) .
Most naturally occurring amino acids are the L form.
You can zoom and rotate the two molecules below. However much you rotate either of them - they will never be identical...
L - Glutamic acid
D - Glutamic acid