1.38 Ionic bonding - know your ions
1.38 Activity. Know your ions.
Students should:
- 1.38 know the charges of common ions listed
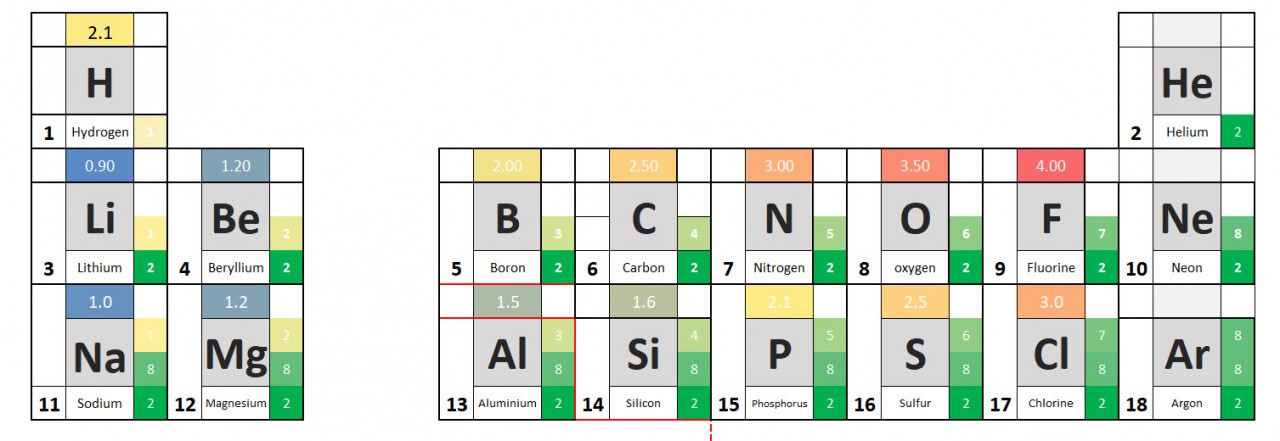
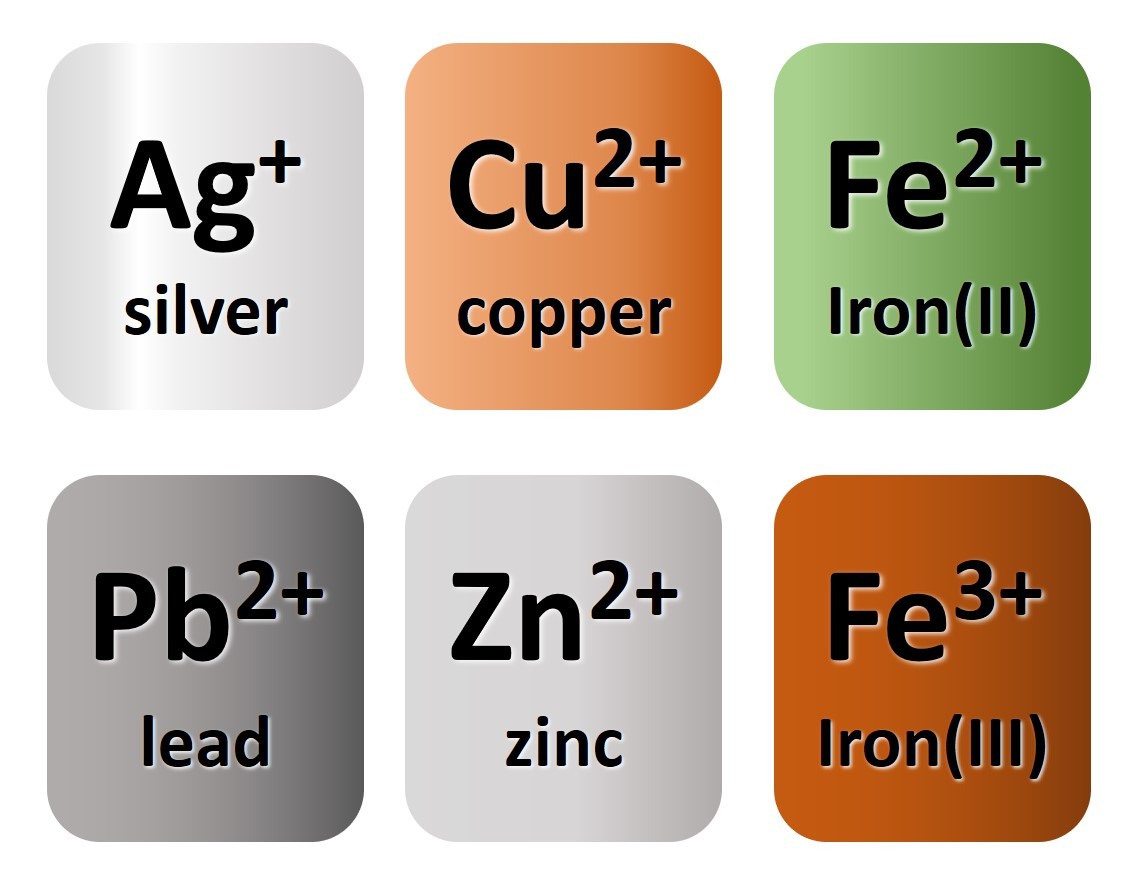
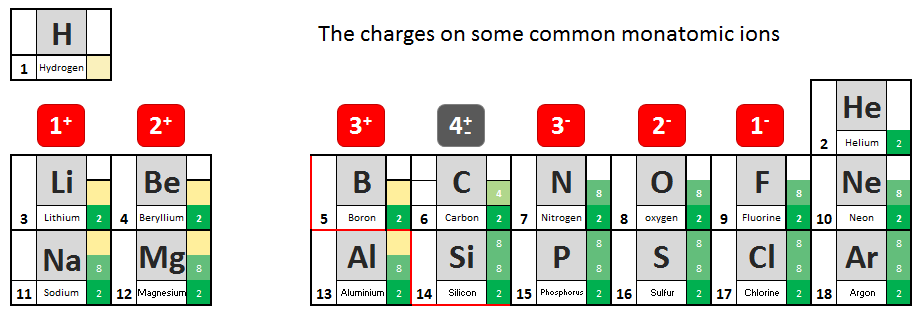
You will have noted from the video that metal atoms tend to lose the electrons in their outermost electrons and form positive ions. The charge of the ion formed is equal to the number of electrons lost.
Non metal atoms gain electrons (when bonding with metals) and form negative ions. The non- metal atom gains enough electrons to complete its outermost shell
Use these ideas and the periodic table given to complete the following exercise.
- Write out the symbols of the first 20 elements in order - as they appear on the periodic table.
- Add the group number to your periodic table.
- From the information given in the video, write down charges on the ions.
- See if you can work out the pattern and add it to your table.
Stay Informed
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.