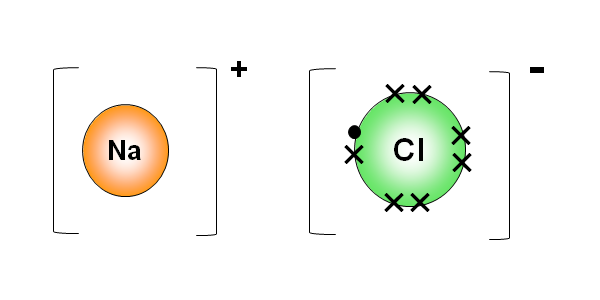1.40 - 1.43 IONIC BONDING - PROPERTIES
| 1.40 draw dot-and-cross diagrams to show the formation of ionic compounds by electron transfer, limited to combinations of elements from Groups 1, 2, 3 and 5, 6, 7 only outer electrons need be shown |
| 1.41 understand ionic bonding in terms of electrostatic attractions |
| 1.42 understand why compounds with giant ionic lattices have high melting and boiling points |
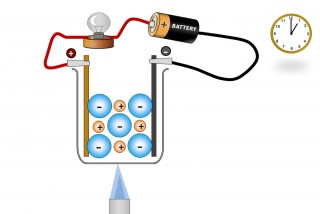
| 1.43 know that ionic compounds do not conduct electricity when solid, but do conduct electricity when molten and in aqueous solution |
Stay Informed
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.