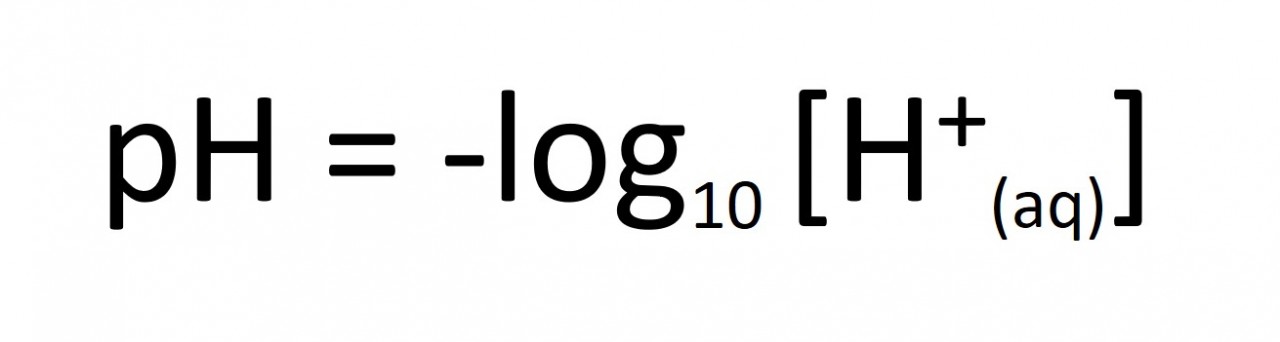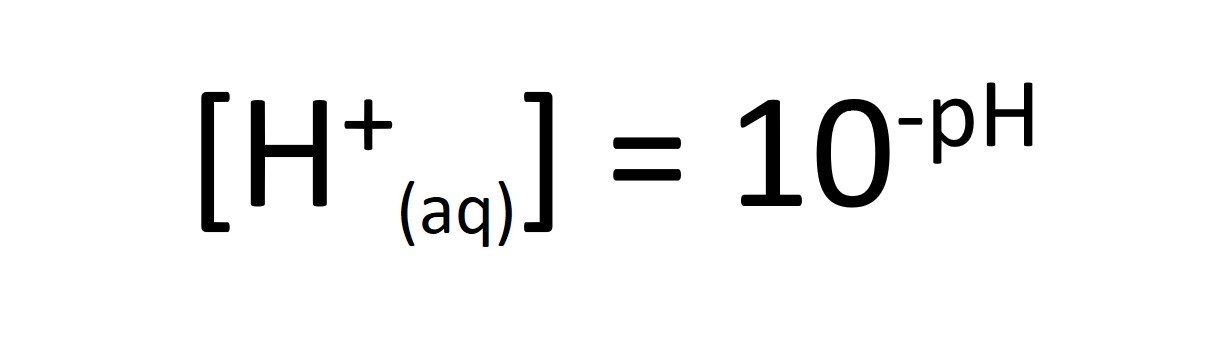1. Macro ( Dilution 10x )
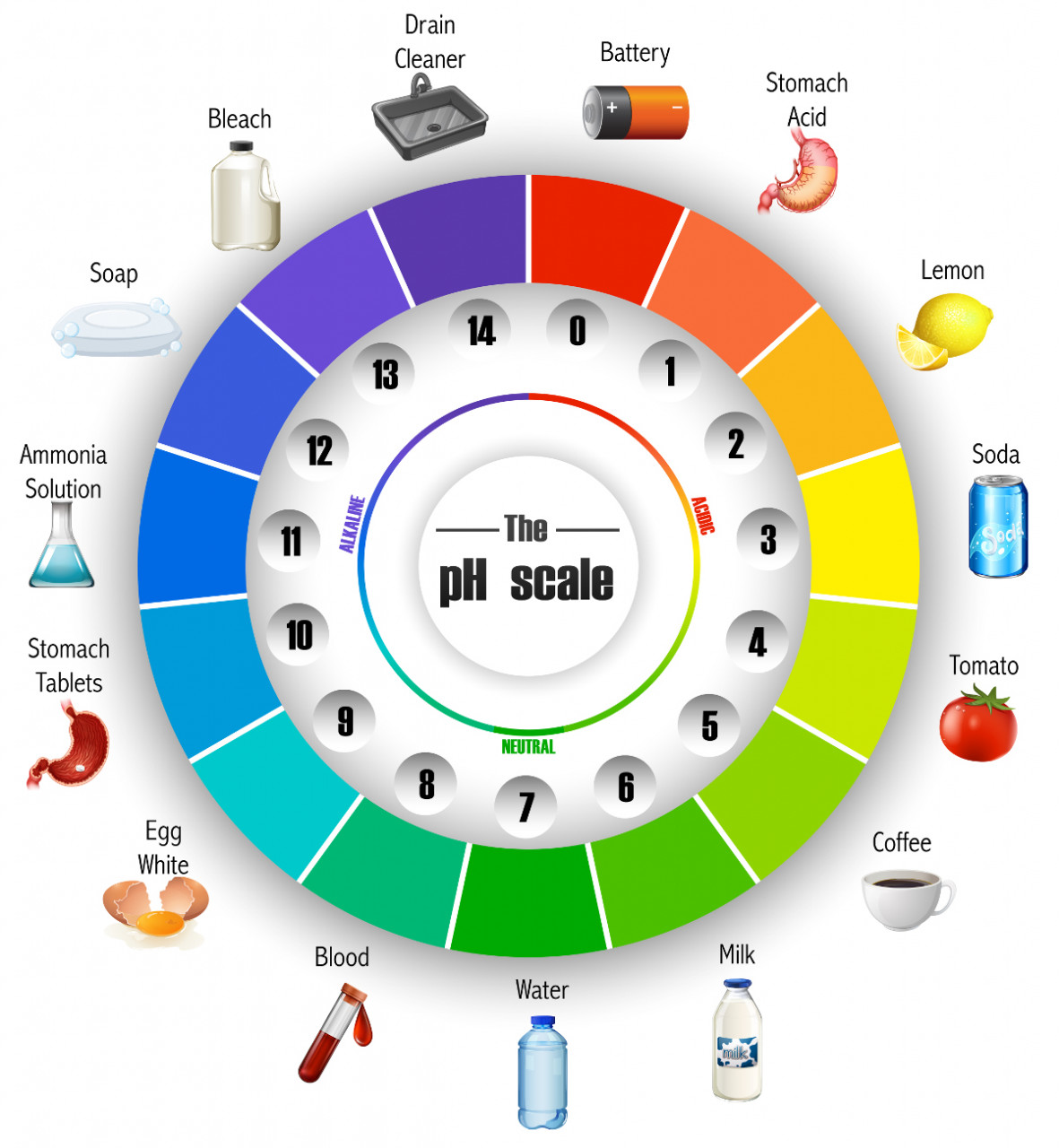
This is useful to simulate the measurement of pH values. You drag the green pH probe into the solution to measure the pH. Try adding battery acid and measure the pH.
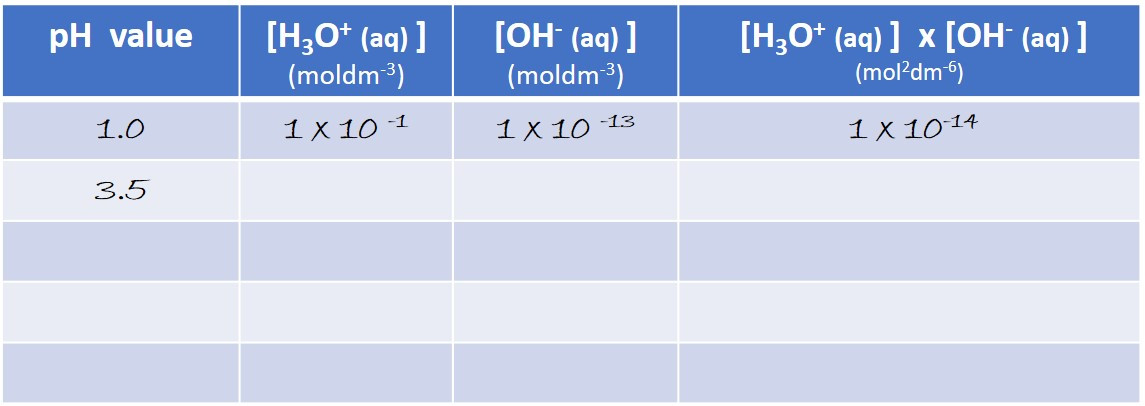
Work out how much you need to dilute the battery acid to increase its pH by one unit...